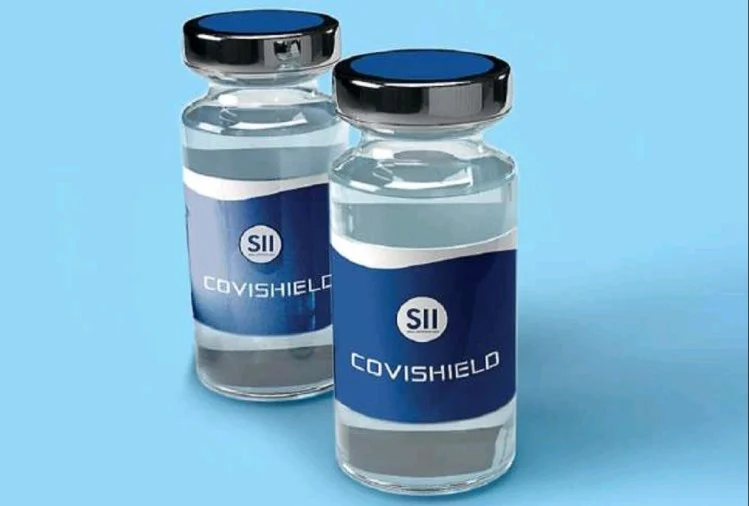
This WHO Medical Product Alert alludes to falsified COVISHIELD (ChAdOx1 nCoV-19 Corona Virus Vaccines
(Recombinant)) recognized in the WHO African Region, and the WHO South-East Asia Region. The adulterated items were accounted for to WHO in July and August 2021. The veritable maker of COVISHIELD (Serum Institute of India Pvt. Ltd.) has affirmed that the items recorded in this alarm are adulterated. These adulterated items have been accounted for at the patient level in Uganda, India and Myanmar.
Authentic COVISHIELD antibody is demonstrated for dynamic vaccination of people 18 years or more established for the avoidance of Covid infection brought about by the SARS-CoV-2 infection. The utilization of certifiable COVID-19 antibodies ought to be as per official direction from public administrative specialists.
Misrepresented COVID-19 immunizations represent a genuine danger to worldwide general wellbeing and spot an extra weight on
weak populaces and wellbeing frameworks. Recognize and eliminate these adulterated items from
course to forestall damage to patients.
The items recognized in this alarm are affirmed as distorted on the premise that they purposely/falsely distort their character, creation or source:
- Cluster 4121Z040 – the expiry date (10.08.2021) on this item is misrepresented
- COVISHIELD 2ml – the certifiable maker doesn’t create COVISHIELD in 2ml (4 dosages).
- Cluster 4126Z079 – the clump number on this item is distorted and the item name: COVISHELD isn’t the right spelling
Table 1: Products subject of WHO Medical Product Alert N°5/2021

Counsel to administrative specialists and people in general
WHO solicitations expanded carefulness inside the stock chains of nations and districts prone to be influenced by these misrepresented items. Expanded cautiousness ought to incorporate medical clinics, facilities, wellbeing focuses, wholesalers, merchants, drug stores, and some other providers of clinical items.
All clinical items should be gotten from approved/authorized providers. The items’ genuineness and state of being ought to be painstakingly checked. Look for counsel from a medical services proficient in the event of uncertainty.
In case you are in control of the above items, kindly don’t utilize them.
On the off chance that you have utilized these items, or you experienced an unfriendly response/occasion having utilized these items, you are encouraged to look for sure fire clinical guidance from a certified medical services proficient and to report the episode to the National Regulatory Authorities/National Pharmacovigilance Center.
Public administrative/wellbeing specialists are encouraged to quickly advise WHO if these distorted items are
found in their country. On the off chance that you have any data concerning the assembling, dispersion, or supply of
these items, kindly contact rapidalert@who.int
Table 2: Photographs of products subject of WHO Medical Product Alert N°5/2021