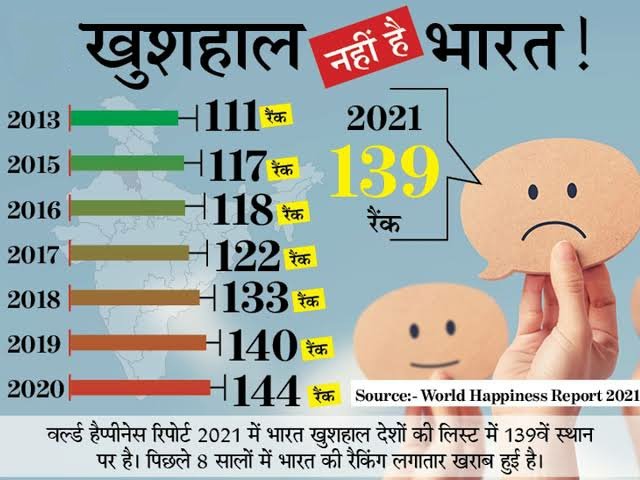
American pharma goliath Johnson and Johnson (J&J) has moved an application to the Central Drugs Standard Control Organization (CDSCO) looking for authorization to direct an investigation of the COVID-19 single shot Janssen immunization in India in youths matured 12 – 17 years.
The single-shot antibody, created by J&J demonstrated to be 85% viability in forestalling serious sickness in Phase 3 human clinical preliminaries.
“Johnson and Johnson is focused on working with worldwide impartial admittance to its COVID-19 immunization and perceive the neglected necessities of youngsters. On August 17, 2021, we presented an application to the Central Drugs Standard Control Organization (CDSCO) to lead an investigation of the Johnson and Johnson COVID-19 immunization in India in young people matured 12 – 17 years,” Johnson and Johnson India representative revealed to Mirror Now.
“To eventually accomplish crowd invulnerability, it is basic that COVID-19 immunization clinical preliminaries keep on pushing ahead in this populace, and we remain profoundly dedicated to the basic work expected to make our COVID-19 antibody fairly open for all age gatherings,” the representative added.
The single shot antibody of J&J is the second COVID-19 immunization which has been conceded Emergency Use Authorisation (EUA) through the most optimized plan of attack endorsement course by the Drug Controller General of India.
Association Health Minister Mansukh Mandaviya had before tweeted about the single shot immunization being allowed the Emergency Use Authorisation in India.
“India grows its antibody bushel! Johnson and Johnson’s single-portion COVID-19 immunization is given endorsement for Emergency Use in India. Presently India has 5 EUA immunizations. This will additionally support our country’s aggregate battle against #COVID19,” Union Minister of Health and Family Welfare Mansukh Mandaviya said in a tweet.
In the period of May, the Drug Controller General of India had allowed Bharat Biotech to lead clinical preliminaries on sound volunteers between 2 to 18 years old for its immunization, Covaxin.
Gujarat-based drug organization Zydus Cadila which is creating DNA antibodies is likewise leading its clinical preliminary for between the age gathering of 12 to 18 years.
The Union government changed the arrangement for administrative endorsements for unfamiliar makers to economically showcase their Covid-19 immunization in the country in April, forgoing the pre-state of stage 2-3 clinical preliminaries for those antibodies that have been allowed crisis endorsements by controllers in the US, EU, UK, and Japan, and those recorded by the WHO.